
GIST Tumors Linked to NF1 Mutations, Genetic Testing Needed
Published Date
By:
- Jackie Carr
Share This:
Article Content
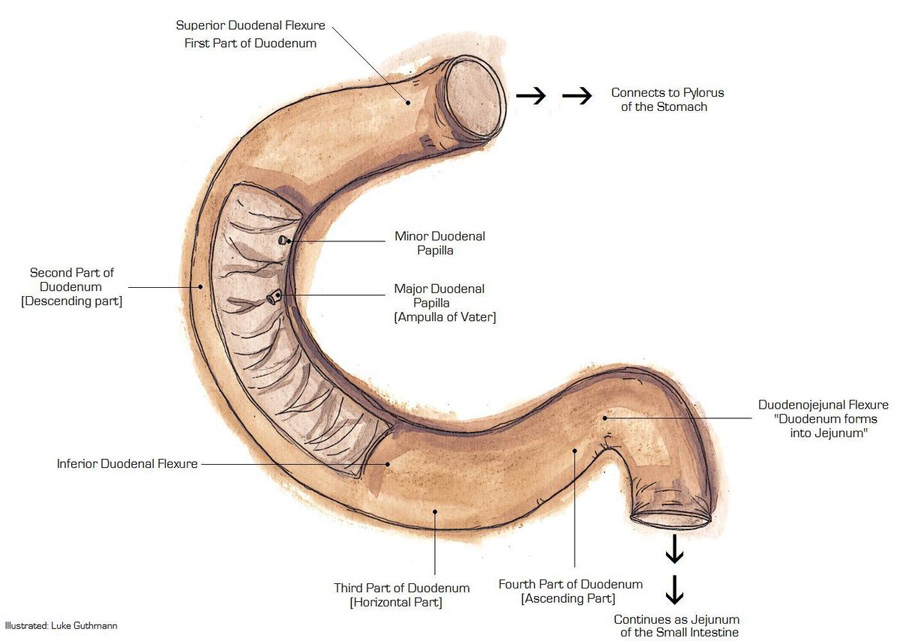
Roughly one-third of gastrointestinal stromal tumors occur in the small bowel, which is comprised of three distinct segments: the duodenum, jejunum and ileum. Of particular note is a section called the duodenal-jejunal flexure where the duodenum bends to become the jejunum. Image courtesy of Luke Guthmann, Wikipedia.
Researchers at UC San Diego Moores Cancer Center, with colleagues from Memorial Sloan Kettering Cancer Center and Fox Chase Cancer Center, have determined that a specific region of the small bowel, called the duodenal-jejunal flexure or DJF, shows a high frequency of gastrointestinal stromal tumors (GISTs) with mutations of the NF1 gene. Results of the study were published in the August online issue of JCO Precision Oncology.
The small bowel, where approximately 30 percent of all GISTs occur, is divided into three anatomically, histologically and functionally distinct segments: the duodenum, jejunum and ileum. Most small bowel GISTs are associated with KIT mutations. However, a subset of GISTs have mutations in other genes, such as NF1.
“Where the duodenum transitions into the jejunum, we are finding an over-representation of NF1-mutated GIST,” said Jason Sicklick, MD, surgical oncologist at Moores Cancer Center.
NF1 can be mutated both somatically (within tumor DNA) or in the germline (part of the hereditary condition called Neurofibromatosis type 1 [NF-1]). Patients with NF-1 are 34 times more likely to develop GIST than unaffected individuals.
“Genomic testing for some of these patients revealed occult germline NF1 mutations with no other obvious clinical symptoms of NF-1,” said Sicklick. “Anyone with a GIST should undergo tumor genetic testing. Currently only 8 to 15 percent of patients get tested. For those patients with NF1 mutations, there are implications for family members of patients who test positive for hereditary NF-1, as they also may be at increased risk of developing cancers, including GIST.”
According to the National Institutes of Health, NF-1 is a condition characterized by changes in skin coloring and the growth of tumors along nerves in the skin, brain and other parts of the body, such as the GI tract. The signs and symptoms of this condition vary widely among affected persons. NF-1 occurs in one in 3,000 to 4,000 individuals worldwide.
GIST represents the most common type of sarcoma in the GI tract, with an annual incidence of 6.8 cases per million people in the United States. These tumors start in special cells in the wall of the GI tract, called the interstitial cells of Cajal (ICCs). ICCs are sometimes dubbed the “pacemakers” of the GI tract because they signal the muscles in the digestive system to contract through peristalsis, moving food and liquid through the system.
Sicklick and colleagues at Moores Cancer Center are searching for a personalized approach to GIST tumors that become progressively resistant to treatment. Ultimately, more than 95 percent of patients with drug-resistant GIST succumb to their cancer, highlighting the necessity for alternative therapeutic targets.
“Patients with GIST should have their tumors profiled with next-generation sequencing panels,” said first author Adam Burgoyne, MD, PhD, medical oncologist at Moores Cancer Center. “We are uncovering a subset of patients, including patients with mutations of KIT, who have downstream mutations that may render them insensitive to conventional targeted therapy.”
Sicklick added: “This insight helps physicians to know which drugs will or won’t work in order to properly treat these deadly tumors. Of critical importance in NF1 mutant GIST, standard-of-care drug regimens aren’t effective.”
Sicklick’s recent GIST research has also identified new gene fusions and mutations associated with subsets of GIST patients. He and his team also provided the first evidence that the Hedgehog signaling pathway is central to the formation of GIST, which are frequently driven by the KIT oncogene.
Moores Cancer Center at UC San Diego Health is the region’s only National Cancer Institute-designated Comprehensive Cancer Center, with nearly 350 medical and radiation oncologists, surgeons and researchers. The center conducts more than 170 open clinical trials, including highly personalized stem cell-based treatments and immunotherapies that boost a patient’s own immune system — the inherent healing powers of the human body.
Co-authors include: Adam M. Burgoyne, Martina De Siena, Maha Alkhuziem, Chih-Min Tang, Paul T. Fanta, John A. Thorson, Lisa Madlensky, Dwayne G. Stupack, and Olivier Harismendy, UC San Diego; Francesco D’Angelo, Sapienza e Universita di Roma; Benjamin Medina, Timothy Bowler, and Ronald P. DeMatteo, Memorial Sloan Kettering Cancer Center; and Martin G. Belinsky and Margaret von Mehren, Fox Chase Cancer Center.
This research was supported by the Tower Cancer Research Foundation, the National Institutes of Health (R01CA102613, R21CA177519, R21CA192072, P30CA023100, U54HL108460, K08CA168999, R21CA192072, P30CA023100), the GIST Cancer Research Fund and Young Gastrointestinal Research Fund.
Share This:
You May Also Like
Stay in the Know
Keep up with all the latest from UC San Diego. Subscribe to the newsletter today.


