UC San Diego Among Multidisciplinary Awards Providing $221M Nationally for Cutting-Edge Projects
Technology & Engineering
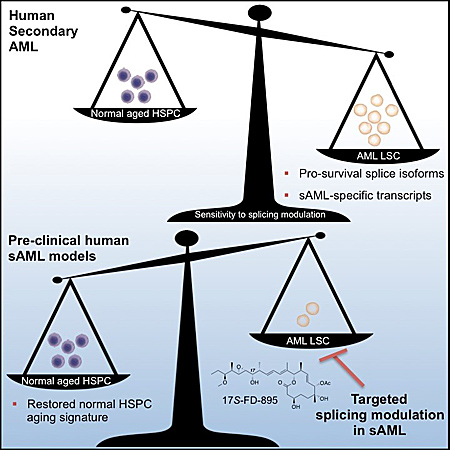
Schematic shows role of RNA splicing in secondary acute myeloid leukemia.
Researchers at University of California San Diego School of Medicine and Moores Cancer Center have identified RNA-based biomarkers that distinguish between normal, aging hematopoietic stem cells and leukemia stem cells associated with secondary acute myeloid leukemia (sAML), a particularly problematic disease that typically afflicts older patients who have often already experienced a bout with cancer.
The findings, published online August 25 in Cell Stem Cell, suggest a new way to predict leukemic relapse early and to identify potential targets for new drug development.
Secondary AML typically follows a chronic pre-malignant disease or treatment for other cancers. Consequently, patients tend to be diagnosed later in life, usually after the age of 60.
“Because of relatively low survival rates and their advancing age, these patients tend to be poor candidates for aggressive therapies, like a bone marrow transplant,” said senior author Catriona Jamieson, MD, PhD, professor of medicine, chief of the Division of Regenerative Medicine at UC San Diego School of Medicine and director of the Stem Cell Research Program at Moores Cancer Center. “There is a pressing need for more effective treatments that prevent disease progression and relapse.”
Aging is a key risk factor for sAML because, over time, hematopoietic stem cells (which give rise to all other blood cell types) accumulate DNA mutations and changes in other molecules that put DNA instructions into action, such as RNA and proteins.
Jamieson’s team wanted to understand how RNA might change with the aging of normal blood stem cells compared with sAML stem cells. “By being able to distinguish benign from malignant aging based on distinctive RNA splicing patterns, we can develop therapeutic strategies that selectively target leukemia stem cells while sparing normal hematopoietic stem cells,” she said.
Leslie Crews, PhD, assistant project scientist in Jamieson’s lab and co-first author with Larisa Balaian, PhD, project scientist, said the team “specifically looked at a process called RNA splicing, which is responsible for removing pieces of extraneous RNA that do not contain instructions to make protein. If disrupted, RNA splicing could enhance the capacity of cells to propagate cancer.”
Using sensitive genetic sequencing technology, the scientists identified unique RNA splicing variants that distinguish normal, aging stem cells from abnormal, malignant ones. “These splicing signatures could potentially be used as clinical biomarkers to detect blood stem cells that show signs of early aging or leukemia, and to monitor patient responses to treatment,” said Crews.
Current AML therapies fail to eliminate dormant leukemia stem cells responsible for disease relapse. “Our findings show that RNA splicing is a unique therapeutic vulnerability for secondary AML,” said Jamieson. “RNA-splicing-targeted therapies may be a potent and selective way to clear leukemia stem cells and prevent relapse.”
The researchers also tested a small molecule splicing modulator compound derived from a natural product and developed in the lab of Michael Burkart, PhD, professor in the Department of Chemistry and Biochemistry at UC San Diego.
In patient-derived animal models, they found that just three doses of the compound, called 17S-FD-895, significantly reduced the ability of leukemia stem cells to self-renew. The authors say it’s the first study to show RNA splicing modulators inhibit cancer stem cell activity.
“While genetic and proteomic tools can address the mechanisms of splicing at a global level, small molecule modulators allow the selective examination of splicing mechanism,” said Burkart. “This work was enabled by our ability to prepare stable analogs of natural products that modulate the spliceosome and represents more than 10 years of effort into synthetic and medicinal chemistry.
“We are optimistic that these findings will support our long term goal of delivering a clinical candidate to combat blood-borne cancer. Furthermore, we see these materials as important probes to dissect the complex yet important mechanics of disease related splicing events.
Crews noted that RNA splicing-targeted agents have been shown to have activity in a variety of solid tumors so the findings may be relevant to a variety of cancers, such as breast and drug-resistant melanoma.
Co-authors include: Nathaniel P. Delos Santos, Heather S. Leu, Angela C. Court, Elisa Lazzari, Anil Sadarangani, Maria A. Zipeto, James J. La Clair, Reymundo Villa, Anna Kulidjian, Sheldon Morris, and Edward D. Ball, all at UC San Diego; and Ranier Storb, Fred Hutchinson Cancer Research Center and University of Washington.
Funding for this research came, in part, from The Leukemia & Lymphoma Society, the Moores Foundation, the Mizrahi Family Foundation, the Sanford Stem Cell Clinical Center, the National Institutes of Health (P30-CA 023100, R21 CA 189705, CA 078902, CA 018029), the Federico Foundation, the UC San Diego AML Research Fund, and the Office of the Assistant Secretary of Defense for Health Affairs.
Keep up with all the latest from UC San Diego. Subscribe to the newsletter today.