By:
- Heather Buschman
Published Date
By:
- Heather Buschman
Share This:
‘A Tornado at the Front Door, a Tsunami at the Back Door’
20-year-old born with rare disease arrives at crossroads: continue toward future most likely leading to early death or become first patient to undergo new gene-and-stem cell therapy developed by School of Medicine researchers

Stephanie Cherqui, (right) developed a unique stem cell-based therapy for cystinosis over years of research at UC San Diego School of Medicine.
For the majority of Jordan Janz’s 20 years of life, most neighbors in his tiny Canadian town never knew he was sick. Janz snowboarded, hunted and fished. He hung with friends, often playing ice hockey video games. He worked in shipping and receiving for a company that makes oil pumps.
But there were times when Janz was younger that he vomited up to 13 times each day. He received a growth hormone injection every day for six years. He needed to swallow 56 pills every day just to manage his symptoms. And the medication required around-the-clock administration, which meant his mother or another family member had to get up with him every night.
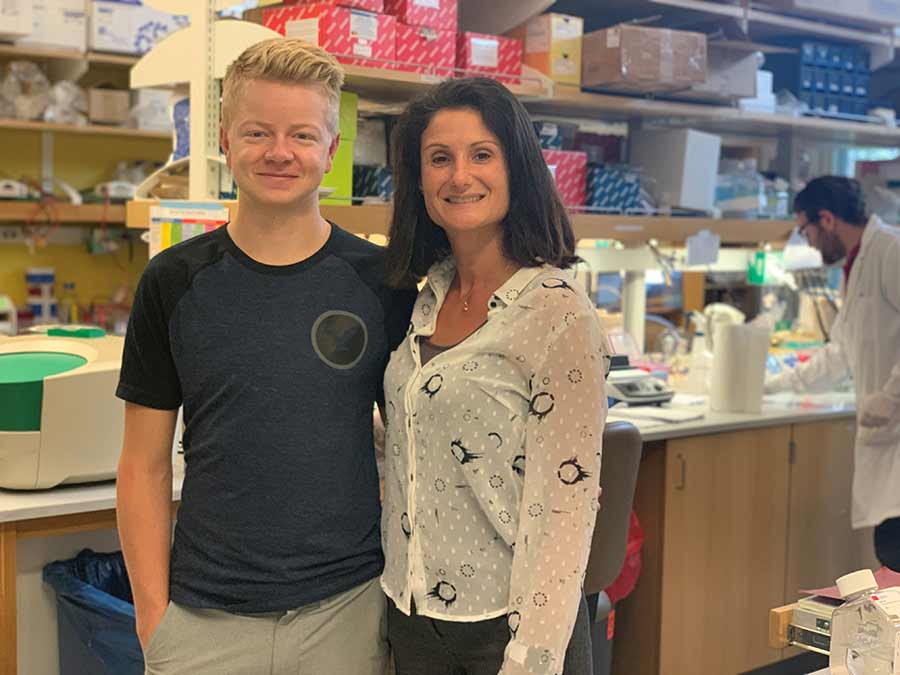
Jordan Janz (left) and Cherqui (right) toured the UC San Diego School of Medicine lab where his therapy was developed, shortly before undergoing treatment as part of a Phase I/II clinical trial.
“I was tired for school every day,” Janz said. “I was held back in second grade because I missed so much school. And because the medication had a bad odor to it, when I did go to school kids would ask, ‘What’s that smell?’ It was hard.”
Janz was born with cystinosis, a rare metabolic disorder that’s detected in approximately one in 100,000 live births worldwide. People with cystinosis inherit a mutation in the gene that encodes a protein called cystinosin. Cystinosin normally helps cells transport the amino acid cystine. Because cells in people with cystinosis don’t produce the cystinosin protein, cystine accumulates. Over the years, cystine crystals build up and begin to damage tissues and organs, from the kidneys and liver to muscles, eyes and brain. Numerous symptoms and adverse consequences result.
These days, Janz manages his condition. There’s a time-release version of the symptom-relieving medication now that allows him to go 12 hours between doses, allowing for a good night’s sleep. But there’s no stopping the relentless accumulation of cystine crystals, no cure for cystinosis.
In October 2019, Janz became the first patient to receive treatment as part of a Phase I/II clinical trial to test the safety and efficacy of a unique gene therapy approach to treating cystinosis. The treatment was developed over more than a decade of research by Stephanie Cherqui, associate professor of pediatrics, and her team at University of California San Diego School of Medicine.
“The day they started looking for people for the trial, my mom picked up the phone, found a number for Dr. Cherqui, called her and put my name in as a candidate,” Janz said.
Janz’s mom, Barb Kulyk, has long followed Cherqui’s work. Like many parents of children with cystinosis, Kulyk has attended conferences, read up on research and met many other families, doctors and scientists working on the condition. Kulyk says she trusts Cherqui completely. But she was understandably nervous for her son to be the first person ever to undergo a completely new therapy.
“It’s like giving birth,” she said shortly before Janz received his gene therapy. “You’re really looking forward to the outcome, but dreading the process.”
The treatment
Cherqui’s gene therapy approach involves genetically modifying the patient’s own stem cells. To do this, her team obtained hematopoietic stem cells from Janz’s bone marrow. These stem cells are the precursors to all blood cells, including both red blood cells and immune cells. The scientists then re-engineered Janz’s stem cells in a lab using gene therapy techniques to introduce a normal version of the cystinosin gene. Lastly, they reinfused Janz with his own now-cystinosin-producing cells. The approach is akin to a bone marrow transplant—the patient is both donor and recipient.
“A bone marrow transplant can be very risky, especially when you take hematopoietic stem cells from a another person. In that case, there’s always the chance the donor’s immune cells will attack the recipient’s organs, so-called graft-versus-host disease,” Cherqui explained. “It’s a great advantage to use the patient's own stem cells.”
As is the case for other bone marrow transplants, Janz’s gene-modified stem cells are expected to embed themselves in his bone marrow, where they should divide and differentiate to all types of blood cells. Those cells are then expected to circulate throughout his body and embed in his tissues and organs, where they should produce the normal cystinosin protein. Based on Cherqui’s preclinical data, she expects the cystinosin protein will be transferred to the surrounding diseased cells. At that point, Janz’s cells should finally be able to appropriately transport cystine for disposal—potentially alleviating his symptoms.
Before receiving his modified stem cells, Janz had to undergo chemotherapy to make space in his bone marrow for the new cells. Not unexpectedly, Janz experienced a handful of temporary chemotherapy-associated side-effects, including immune suppression, hair loss and fatigue. He also had mucositis, an inflammation of mucous membranes lining the digestive tract, which meant he couldn’t talk or eat much for a few days.
Now, only three months after his transfusion of engineered stem cells, Cherqui reports that Janz is making a good recovery, though it’s still too early to see a decrease in his cystinosis-related symptoms.
“I’ve been sleeping at least 10 hours a day for the last few weeks,” Janz said. “It’s crazy, but I know my body is just working hard to, I guess, create a new ‘me.’ So it's no wonder I'm tired. But I'm feeling okay overall.
“One of the hardest parts for me is being inactive for so long. I’m not used to doing nothing all day. But I’m taking an online course while I wait for my immune system to rebuild. And I’m getting pretty good at video games.”
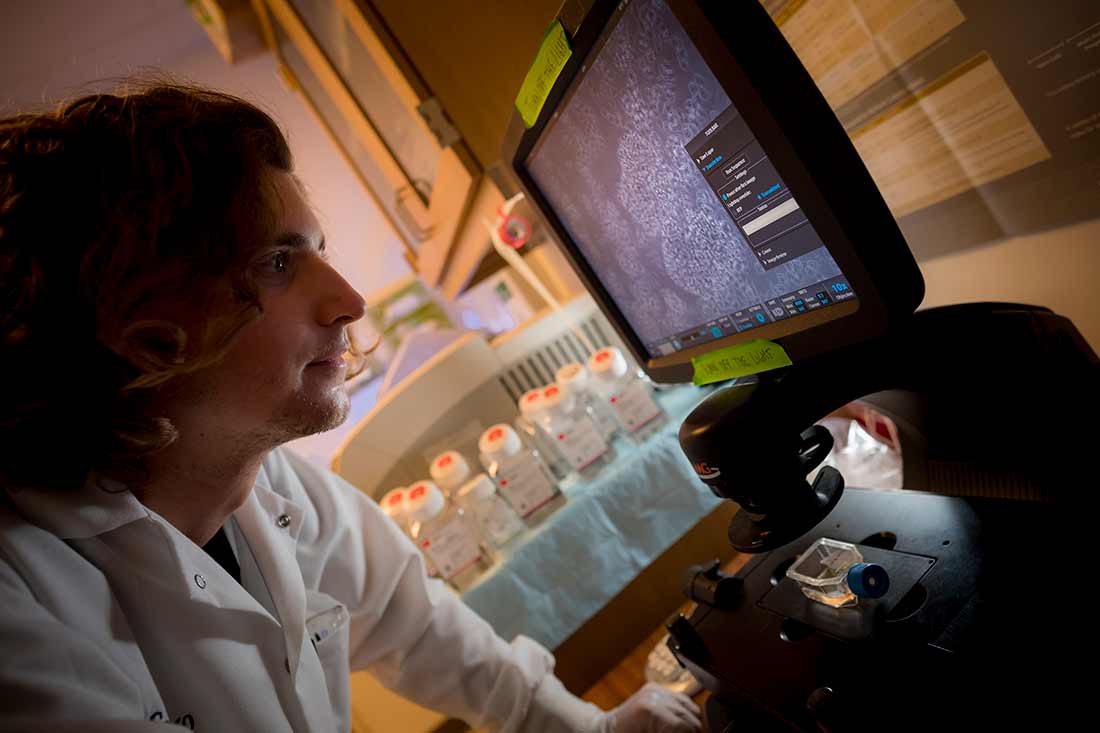
Joseph Rainaldi, a member of Cherqui’s lab, analyzes Janz’s blood cells to measure his levels of cystine and determine how well the therapy is working.
Like all Phase I/II clinical trials, the current study is designed to first test the safety and tolerability of the new treatment. Janz knows the treatment might not necessarily help him.
“When we started this trial, my mom explained it like this: ‘We have a tornado at the front door and a tsunami at the back door, and we have to pick one to go through. Neither will be any fun and we don’t know what’s going to happen, but you have to believe you will make it and go.
“So we weighed the pros and cons and, basically, if I don’t do this trial now, when I’m older I might not be healthy and strong enough for it. So I decided to go for it because, even if there are consequences from the chemotherapy, if it works I could live 20 years longer than I’m supposed to and be healthy for the rest of my life. That’s worth it.”
Besides the possible benefit to himself, Janz also sees his participation in the clinical trial as a way to contribute to the tight-knit community of families with children who have cystinosis.
“I’m willing to do if it helps the kids,” he said. “Somebody has to do it. I don’t have the money to donate to scientific conferences and stuff like that, but I can do this trial.”
The trial
If the treatment continues to meet certain criteria for safety and efficacy for Janz and one other participant after three months, two more adult participants will be enrolled. Three months after that, if the treatment continues to be safe and effective, the trial might enroll two adolescent participants. To participate in the clinical trial, individuals must meet specific eligibility requirements.
Later in the trial, Cherqui and team will begin measuring how well the treatment actually works. The specific objectives include assessing the degree to which gene-modified stem cells establish themselves in bone marrow, how they affect cystine levels and cystine crystal counts in blood and tissues.
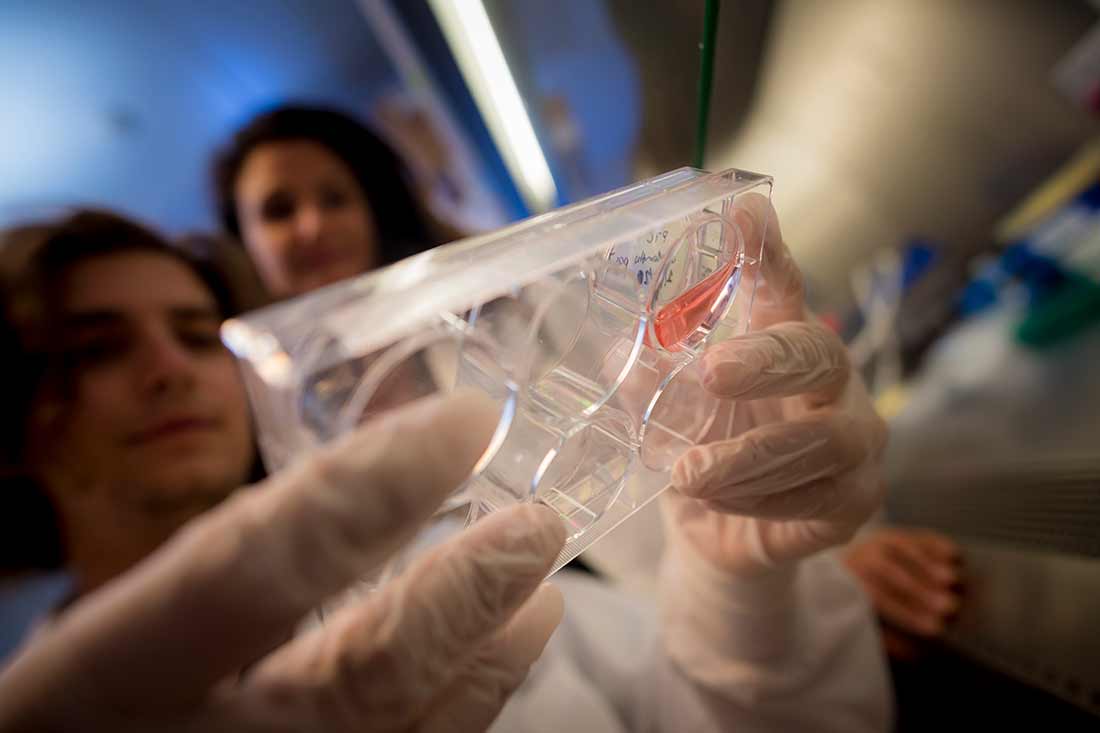
Janz’s blood and kidney cells are collected in Cherqui’s lab for analysis, as part of the clinical trial.
“This trial is the first to use gene-modified hematopoietic stem cell gene therapy to treat a multi-organ degenerative disorder for which the protein is anchored in the membrane of the lysosomes, as opposed to secreted enzymes,” Cherqui said. “We were amazed when we tested this approach in the mouse model of cystinosis—autologous stem cell transplantation reversed the disease. The tissues remained healthy, even the kidneys and the eyes.”
Trial participants are closely monitored for the first 100 days after treatment, then tested again at six, nine, 12, 18 and 24 months post-gene therapy for a variety of factors, including vital signs, cystine levels in a number of organs, kidney function, hormone function and physical well-being.
“If successful in clinical trials, this approach could provide a one-time, lifelong therapy that may prevent the need for kidney transplantation and long-term complications caused by cystine buildup,” Cherqui said.
The future
For the trial participants, all of the pre-treatment tests, the treatment itself, and monitoring afterward means a lot of travel to and long stays in San Diego.
It’s tough on Kulyk and Janz. They have to fly in from Alberta, Canada and stay in a San Diego hotel for weeks at a time. Kulyk has two older adult children, as well as a 12-year-old and a 7-year-old at home.
“I've missed a lot of things with my other kids, but none of them seem to hold any grudges,” she said. “They seem to be totally fine and accepting. They’re like, ‘We’re fine, mom. You go and take care of Jordan.’”
Janz is looking forward to getting back home to his friends, his dog and his job, which provided him with paid leave while he received treatment and recovers.
For Cherqui, the search for a cystinosis cure is more than just a scientific exercise. Cherqui began working on cystinosis as a graduate student more than 20 years ago. At the time, she said, it was simply a model in which to study genetics and gene therapy.
“When you read about cystinosis, it’s just words. You don’t put a face to it. But after I met all the families, met the kids, and now that I’ve seen many of them grow up, and some of them die of the disease—now it’s a personal fight, and they are my family too.”
Patients with cystinosis typically experience kidney failure in their 20s, requiring kidney dialysis or transplantation for survival. For those born with cystinosis who make it into adulthood, the average lifespan is approximately 28 years old.
“I’m optimistic about this trial because it’s something we’ve worked so hard for and now it’s actually happening, and these families have so much hope for a better treatment,” Cherqui said. “After all the years of painstaking laboratory research, we now need to move into the clinic. If this works, it will be wonderful. If it doesn’t, we will all be disappointed but a least we’ll be able to say we tried.”

Janz (center) and his younger siblings.
Nancy Stack, who founded the Cystinosis Research Foundation after her own daughter, Natalie, was diagnosed with the disease, calls Cherqui “the rock star of our community.”
“She cares deeply about the patients and is always available to talk, to explain her work and to give us hope,” Stack said. “She said years ago that she would never give up until she found the cure—and now we are closer to a cure than ever before.” (Read more about Natalie here.)
In addition to cystinosis, Cherqui says this type of gene therapy approach could also lead to treatment advancements for other multi-organ degenerative disorders, such as Friedreich’s ataxia and Danon disease, as well as other kidney, genetic and systemic diseases similar to cystinosis.
While they wait for the long-term results of the treatment, Kulyk is cautiously hopeful.
“Moms are used to being able to fix everything for their children—kiss boo-boos make them better, make cupcakes for school, whip up Halloween costumes out of scraps, pull a coveted toy out of thin air when it has been sold out for months.
“But we have not been able to fix this, to take it away. I not only want this disease gone for my child, I want cystinosis to be nothing more than a memory for all the children and adults living with it. I know that even if and when Jordan is cured, there will still be so much work to do, in terms of regulatory approvals and insurance coverage.
“Having hope for your child’s disease to be cured is a slippery slope. We have all been there, held hope in our hands and had to let go. But, I find myself in a familiar place, holding onto hope again and this time I am not letting go.”
For more information about the Phase I/II clinical trial for cystinosis and to learn how to enroll, call 1-844-317-7836 or email alphastemcellclinic@ucsd.edu.
Cherqui’s research has been funded by the Cystinosis Research Foundation, California Institute for Regenerative Medicine (CIRM), and National Institutes of Health. She receives additional support from the Sanford Stem Cell Clinical Center and CIRM-funded Alpha Stem Cell Clinic at UC San Diego Health, and AVROBIO.
Share This:
You May Also Like
Stay in the Know
Keep up with all the latest from UC San Diego. Subscribe to the newsletter today.