Surprising Beauty Found in Bacterial Cultures
E. coli and A. baylyi form intricate flower patterns under the microscope
Published Date
By:
- Michelle Franklin
Share This:
Article Content
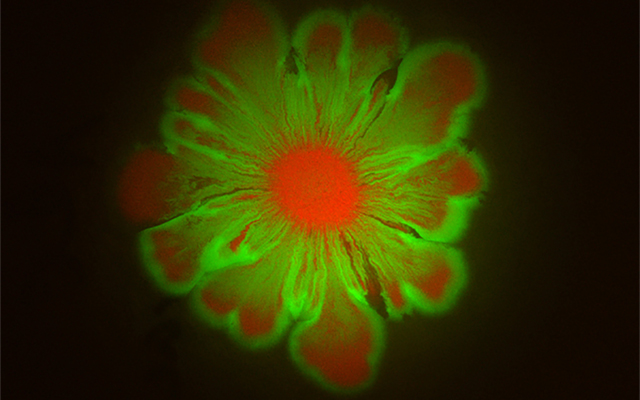
Microbial communities inhabit every ecosystem on Earth, from soil to rivers to the human gut. While monoclonal cultures often exist in labs, in the real world, many different microbial species inhabit the same space. Researchers at University of California San Diego have discovered that when certain microbes pair up, stunning floral patterns emerge.
In a paper published in a recent issue of eLife, a team of researchers at UC San Diego’s BioCircuits Institute (BCI) and Department of Physics, led by Research Scientist and BCI Associate Director Lev Tsimring, reports that when non-motile E. coli (Escherichia coli) are placed on an agar surface together with motile A. baylyi (Acinetobacter baylyi), the E. coli “catch a wave” at the front of the expanding A. baylyi colony.
The agar provided food for the bacteria and also a surface on which E. coli couldn’t easily move (making it non-motile). A. baylyi, on the other hand, can crawl readily across the agar using microscopic legs called pili. Thus, a droplet of pure E. coli would barely spread over a 24-hour period, while a droplet of pure A. baylyi would cover the entire area of the petri dish.
Yet when the E. coli and A. baylyi were mixed together in the initial droplet, both strains flourished and spread across the whole area as the non-motile E. coli hitched a ride on the highly mobile A. baylyi. However, what most surprised researchers were intricate flower-like patterns that emerged in the growing colony over a 24-hour period.
“We were actually mixing these two bacterial species for another project, but one morning I found a mysterious flower-like pattern in a petri dish where a day earlier I placed a droplet of the mixture. The beauty of the pattern struck me, and I began to wonder how bacterial cells could interact with each other to become artists,” said Liyang Xiong, Ph.D. ’19, who was a graduate student in the Physics Department and is the lead author of the study.
To uncover how the flower patterns were formed, Xiong et al. developed mathematical models that took into account the different physical properties of the two strains, primarily the differences in their growth rate, motility and effective friction against the agar surface. The theoretical and computational analysis showed that the pattern formation originates at the expanding boundary of the colony, which becomes unstable due to drag exerted by the E. coli that accumulate there.
In areas where there is less E. coli accumulation, there is also less friction, allowing the boundaries to push out faster. In the areas where there is more E. coli accumulation and more friction, the boundaries stagnate. This is what creates the “petals” of the flower.
Further analysis suggests this type of pattern is expected to form when motile bacteria are mixed with a non-motile strain that has a sufficiently higher growth rate and/or effective surface friction, which could have important implications in studying growing biofilms.
Biofilms are communities of microorganisms—including bacteria and fungi—that adhere to each other and to surfaces, creating strong matrices that are difficult to break down. Common examples include dental plaque and pond scum. They also grow in medical devices such as pacemakers and catheters. Learning how non-motile bacteria can “stick” to motile bacteria may provide insight into how biofilms are formed and how they can be eliminated.
“Bacterial pattern formation has been an active area of research in the last few decades,” said Lev Tsimring, “However, the majority of laboratory studies and theoretical models were focused on the dynamics of single-strain colonies. Most bacteria in natural habitats live in multi-strain communities, and researchers are finally beginning to look for mechanisms controlling their co-habitation. While a number of biochemical mechanisms of inter-species communication and cooperation have been identified, we found that surprising complexity may result from purely physical interaction mechanisms.”
The BioCircuits Institute (BCI) is a multidisciplinary research unit that focuses on understanding the dynamic properties of biological regulatory circuits that span the scales of biology, from intracellular regulatory modules to population dynamics and organ function. BCI seeks to develop and validate theoretical and computational models to understand, predict and control complex biological functions. The institute is comprised of over 50 faculty from UC San Diego and other local institutions, including Scripps Research, the Salk Institute and the Sanford-Burnham Medical Research Institute.
Other researchers involved in this project are Robert Cooper, Jeff Hasty, Yuansheng Cao and Wouter-Jan Rappel, all with UC San Diego. This work was supported by the National Institutes of Health (grant R01-GM069811), the National Science Foundation (grant PHY-1707637), San Diego Center for Systems Biology (NIH grant P50-GM085764) and the DOD Office of Naval Research (grant N00014-16-1-2093).
Share This:
You May Also Like
Stay in the Know
Keep up with all the latest from UC San Diego. Subscribe to the newsletter today.