
Chemists, Physicists Create Spongy Droplets to Mimic Cellular Organelles
Published Date
By:
- Cynthia Dillon
Share This:
Article Content
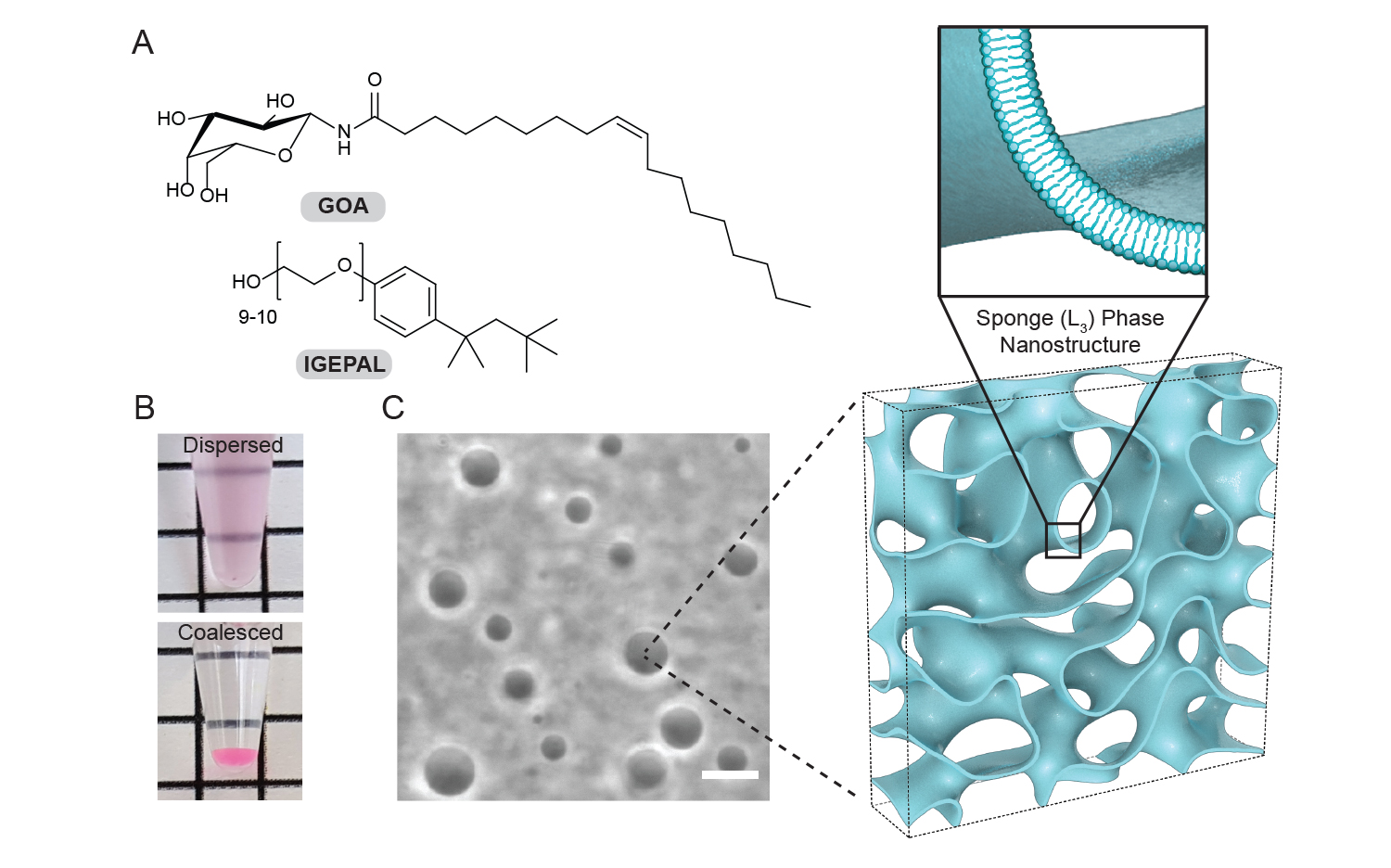
Physical characterization of the lipid sponge droplets. Intensity profile from a droplet dispersion: A) Chemical structures of N-oleoyl β-D-galactopyranosylamide (GOA) and octylphenoxypolyethoxyethanol (IGEPAL) – the principal constituents of the sponge droplets B) A freshly prepared dispersion of sponge droplets appear turbid. On long standing, the droplets merge into a single dense phase at the bottom of the tube. (C) Phase-contrast image of a typical droplet dispersion and illustration of the droplets’ porous, bilayer-rich nanostructure. Image by Ahanjit Bhattacharya.
A team of scientists at UC San Diego led by Professor of Chemistry and Biochemistry Neal Devaraj work to create artificial materials that mimic the functions of living cells. Ideally the synthetic cells do the same things natural cells do—things like segregating molecules and biochemical reactions using compartments known as organelles.
If you think of a living cell as a busy factory, an organelle would be a room within the factory walls with special conditions where certain functions are performed. In the human body, there are many membrane-enclosed organelles. One of those “special rooms” is the endoplasmic reticulum, or ER. It contains highly interconnected and dense membrane networks that enable a large number of reactions using a number of entrapped enzymes.
Using a recently discovered sponge-like fatty droplet, Devaraj and a team that included Ahanjit Bhattacharya and Henrike Niederholtmeyer, co-first authors of the research paper, were able to mimic the functions of an ER-like organelle. These lipid structures, as described in the Proceedings of the National Academy of Sciences, could serve as additional models for protocellular evolution and as tools for scaffolding biochemical reactions in a cell-free system.
Taking a bottom-up approach to synthetic biology, the UC San Diego chemists with Professor of Physics Sunil Sinha showed that the lipid sponge droplets can be programmed to internally concentrate specific proteins, host and accelerate biochemical transformations and to rapidly and reversibly sequester and release proteins to control enzymatic reactions.
Bhattacharya explained that the open and liquid nature of the droplets allows exchange with the surrounding environment, as well as reversible, light-responsive control over protein localization and concentration, which is difficult to engineer in a vesicle-based artificial organelle.
“Spatial organization is likely essential for expanding the biochemical functions of synthetic reaction systems, including artificial cells,” said Devaraj. “Many studies have attempted to mimic organelle functions using certain membrane-bound vesicles. However, vesicles typically suffer from highly limited transport across the membranes and an inability to mimic the dense membrane networks typically found in organelles such as the endoplasmic reticulum.”
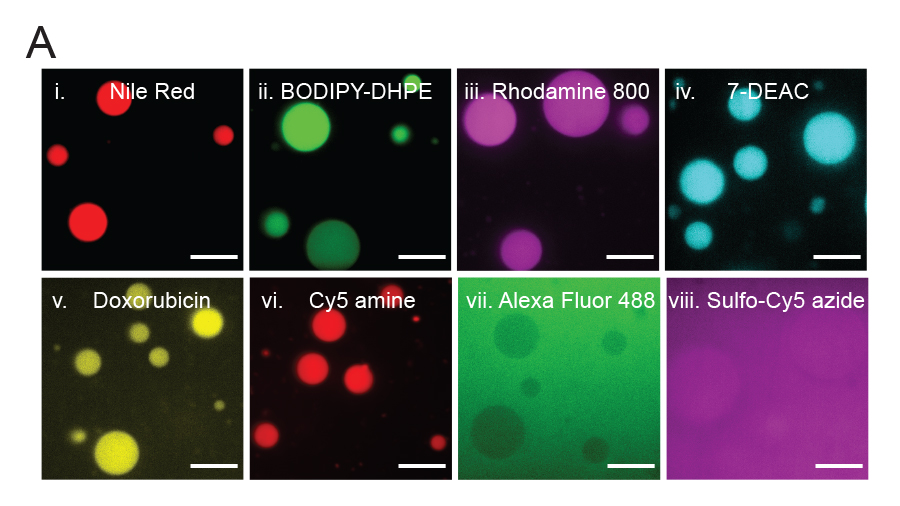
Partitioning of various molecules into lipid sponge droplets. (A) Fluorescent molecules having poor or limited solubility in water, such as Nile Red (i) BODIPY-DHPE (ii) Rhodamine 800 (iii) 7-diethylaminocoumarin (iv) doxorubicin (v) and Cy5 amine (vi). Highly water soluble molecules such as Alexa Fluor 488 (vii) and sulfo-Cy5 azide (viii) are not partitioned and hence may be used as fluorescent labels for macromolecules to study their partitioning behavior unambiguously. Image by Ahanjit Bhattacharya and Henrike Niederholtmeyer.
The researchers concluded that the liquid sponge droplets—with their versatile, self-assembled and programmable nature—will find application as organelle-mimics to spatially organize biochemical reactions within artificial cellular systems and in cell-free systems.
“In addition to helping us understand the role of compartmentalization in biology, lipid sponge droplets may be used to improve cell-free synthesis of commodity biologics, and could also have future applications in drug delivery and sensing,” said Devaraj.
This research is supported by the National Science Foundation (CHE-1844346); the Department of Defense (Army Research Office) through the Multidisciplinary University Research Initiative (Award W911-NF-13-1-0383); the U.S. Department of Energy, Biomolecular Materials Program, Division of Materials Sciences and Engineering (DE-SC0018086); a Swiss National Science Foundation fellowship (P300PA_174346); Advanced Photon Source, Argonne National Laboratory; UC San Diego; University of Freiburg; TU Dresden; UC Irvine Materials Research Institute; Northwestern University’s NUANCE Center, which has received support from the Soft and Hybrid Nanotechnology Experimental Resource (NSF ECCS-1542205); the MRSEC program (NSF DMR-1720139) at the Materials Research Center; the International Institute for Nanotechnology (IIN) and the State of Illinois, through the IIN.
Share This:
Stay in the Know
Keep up with all the latest from UC San Diego. Subscribe to the newsletter today.


