Not So Great Expectations: Pain in HIV Related to Brain’s Expectations of Relief
Neuroimaging study reveals potential brain mechanism underlying chronic neuropathic pain in individuals with HIV
By:
- Nicole Mlynaryk, Bigelow Science Communication Fellow
Published Date
By:
- Nicole Mlynaryk, Bigelow Science Communication Fellow
Share This:
Article Content
As medical advances help individuals with HIV survive longer, there is an increasing need to treat their chronic symptoms. One of the most common is neuropathic pain, or pain caused by damage to the nervous system.
Distal sensory polyneuropathy (DSP) is the most prevalent neurological problem in HIV infection, affecting 50 percent of all HIV patients. Most persons with DSP describe sensations of numbness, tingling, burning and stinging in their hands or feet, which impair daily functioning and can lead to unemployment and depression.
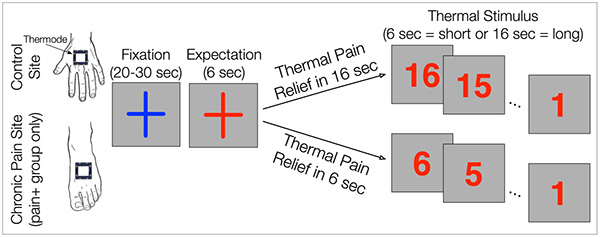
HIV patients with and without chronic neuropathic pain received short or long heat stimuli on their hands (control site) or feet (neuropathic site).
Previous research on DSP has mostly focused on the peripheral nervous system, but nerve injury cannot fully explain the wide variability in DSP symptoms. Researchers at University of California San Diego School of Medicine and University of California San Francisco instead looked at the brain to see how it may be contributing to patients’ pain.
In a new study, published online October 29, 2021 in Brain Communications, the team observed unique patterns of brain activity in HIV-DSP patients when they experienced a painful stimulus. Compared to other patients with HIV, those with DSP showed increased activity in the anterior insula, a brain area involved in predicting and emotionally processing pain.
“The anterior insula is trying to predict the future for you,” said senior author Alan Simmons, PhD, professor of psychiatry at UC San Diego School of Medicine and research scientist at the Veterans Affairs San Diego Healthcare System. “It’s forming expectations about what is about to happen to you and how you’re going to feel. These expectations of pain play an important role in determining how much pain you then actually experience.”
Participants in the study received painful thermal stimuli on their feet or hands while their brain activity was measured using functional magnetic resonance imaging (fMRI). A visual cue let them know when a painful stimulus was coming, and once it began, a numerical countdown displayed how much longer the stimulus would last. Heat was delivered for either six or 16 seconds at a temperature that participants deemed to be similarly painful in prior tests.
First author Irina Strigo, PhD, professor of psychiatry at UC San Francisco and research biologist at the San Francisco Veterans Affairs Health Care System, compared the patients’ brain activity at the start of the short and long pain trials. The stimulus intensity was no different in either case, but when patients knew it was going to last longer, the anterior insula became more active. This enhanced insular activity and reduced pain relief was also specific to the trials when pain was delivered to the neuropathic limb.
“This lets you know that a key aspect of pain in neuropathy is your reaction to the idea that the pain is going to last,” said Simmons. “If you know the pain will be short, you brace and absorb it but it doesn’t dramatically burden you. On the other hand, if you learn the pain will be ongoing and inescapable, your brain will react to that and perceive the pain as overwhelming.”
Simmons suggests that these patients’ brains have been conditioned by their repeated pain to become increasingly emotionally distressed by it, and these differences in how they perceive their pain and expect it to manifest contribute to why it becomes a chronic experience.
What does this mean for HIV-DSP patients? Another finding in the study may hint at a solution. The researchers found a different pattern in the patients’ anterior cingulate cortex, a brain area involved in regulating subjective feelings of pain unpleasantness. The higher their cingulate activity was, the less they said their pain interfered with their daily lives. Reducing anterior insula activity or increasing cingulate activity may thus improve pain outcomes for HIV-DSP patients.
“Once the pain signal reaches the brain, the insula starts telling you, ‘This is going to be long and overwhelming,’ but the cingulate can turn down the dial on that,” said Simmons. Physicians may eventually use advanced tools to modulate brain activity in these areas, but in the meantime, Simmons says simple breathing and mindfulness practices may help.
“Perhaps even just being more aware that your emotions and expectations affect your experience of pain will help people engage these regulators in their brain.”
Co-authors include: John Keltner and Ronald Ellis, UC San Diego.
Funding for this research came, in part, from the United States Department of Veterans Affairs (grants I01-CX-000816, I01-CX001652 and I01-CX001542), the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (grant U19AR076737) and the Painless Research Foundation.
Share This:
You May Also Like
Stay in the Know
Keep up with all the latest from UC San Diego. Subscribe to the newsletter today.