
Researchers Improve Performance of Cathode Material by Controlling Oxygen Activity
Published Date
By:
- Liezel Labios
Share This:
Article Content

SEM image of lithium-rich cathode particles treated with a carbon dioxide-based gas mixture to introduce oxygen vacancies on the surface. Image courtesy of Laboratory for Energy Storage and Conversion, UC San Diego.
An international team of researchers has demonstrated a new way to increase the robustness and energy storage capability of a particular class of “lithium-rich” cathode materials—by using a carbon dioxide-based gas mixture to create oxygen vacancies at the material’s surface. Researchers said the treatment improved the energy density—the amount of energy stored per unit mass—of the cathode material by up to 30 to 40 percent.
The discovery sheds light on how changing the oxygen composition of lithium-rich cathode materials could improve battery performance, particularly in high-energy applications such as electric vehicles. The findings were published July 1 in Nature Communications.
“We’ve uncovered a new mechanism at play in this class of lithium-rich cathode materials. With this study, we want to open a new pathway to explore more battery materials in which we can control oxygen activity,” said Shirley Meng, nanoengineering professor at the University of California San Diego and one of the principal investigators of the study.
Meng leads the Laboratory for Energy Storage and Conversion and is the director of the Sustainable Power and Energy Center, both at UC San Diego. A hallmark of her group’s research efforts is understanding the science behind battery materials—at the level of single atoms and molecules, and at the interfaces. Her group is one of the first to focus on the activity of oxygen atoms in battery materials. Typically, the focus has centered on lithium and transition metal atoms. “Now we’re showing that oxygen also plays a significant role in battery performance,” Meng said.
In the new study, Meng’s group collaborated with researchers from the Chinese Academy of Sciences to develop a way to introduce oxygen vacancies in a class of cathode materials known as lithium-rich layered oxides. These materials have been gaining popularity among battery researchers because they can potentially house more energy than other cathode materials. But lithium-rich cathode materials also have their drawbacks, including slow discharge rates and an issue called voltage fade, which is characterized by a drop in cell voltage with each charge-discharge cycle.
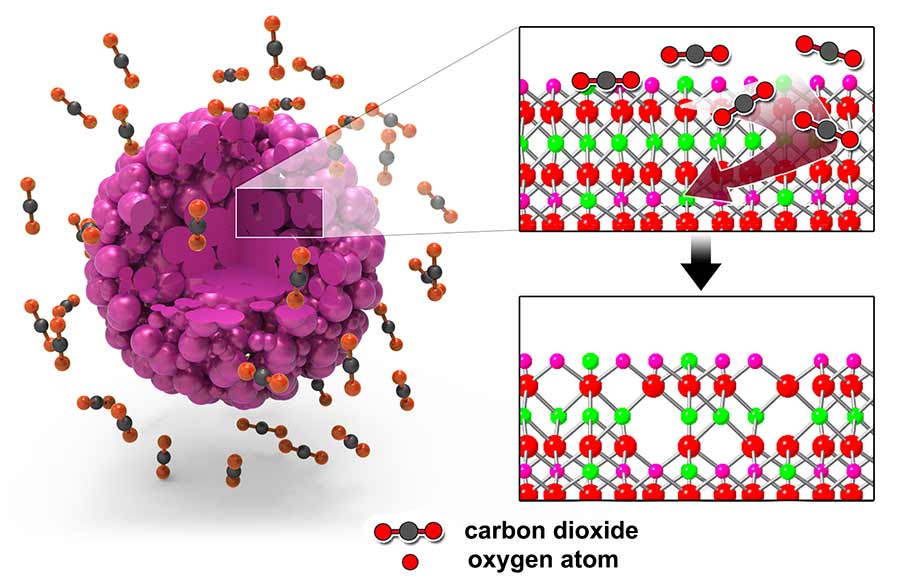
Left: The surface of a lithium-rich cathode particle is treated with carbon dioxide gas. Right: Carbon dioxide gas molecules extract oxygen atoms from the lattice of the lithium-rich cathode particle to create oxygen vacancies at the surface. Image courtesy of Laboratory for Energy Storage and Conversion, UC San Diego.
“We’re presenting a new way to mitigate the issues plaguing lithium-rich cathode materials—through understanding and controlling how oxygen behaves in these materials,” Meng said.
The team found that treating the lithium-rich cathode particles with a carbon dioxide-based gas mixture created oxygen vacancies uniformly throughout the surface of the particles. The treatment only left oxygen vacancies within the first 10 to 20 nanometers without altering the rest of the material’s atomic structure.
“This is a mild treatment that allows us to make controlled changes in the material exactly where we want—near the interface,” said Minghao Zhang, co-first author of the paper and a PhD student at the Jacobs School of Engineering at UC San Diego working in Meng’s group.
In electrochemical tests, the treated material exhibited a relatively high discharge capacity (300 milliamp-hours per gram) with minimal voltage loss after 100 charge-discharge cycles.
“This is a significant improvement with regards to the voltage fade problem, but there’s still a lot of work left to completely resolve this problem,” Meng said.
Through characterization studies in collaboration with groups from Brookhaven National Laboratory and Oak Ridge National Laboratory, researchers provided several reasons why oxygen vacancies improved the cathode material’s performance. They explained that the vacancies allow lithium ions to move around more easily throughout the cathode, leading to high discharge capacity and faster discharge rates. The vacancies also increase the material’s stability by inhibiting the formation of highly reactive oxygen radicals at the cathode material’s surface, which are typically responsible for degrading the electrolyte while the battery is operating. This could mean longer battery lifetime, researchers said.
Illustration of a “spiderweb” for evaluating battery performance based on multiple parameters.
“We can controllably utilize oxygen activity to improve the performance of the material and better control how it works inside the battery,” Zhang said.
As a next step, researchers will work on scaling up the treatment reported in this study. They will also conduct further studies on the oxygen activity in other materials and how it could be leveraged to improve battery performance.
“But before we can decide if this is a promising step forward for batteries, we need to probe whether our technology can improve battery performance based on multiple metrics at once, not just whether it improves a single parameter,” Meng said. “We need to think of improving battery performance like we’re expanding on a spiderweb with multiple variables.”
Full paper: “Gas-solid interfacial modification of oxygen activity in layered oxide cathodes for lithium-ion batteries.” Authors of the study are Minghao Zhang,* Danna Qian, Haodong Liu, Sunny Hy and Ying Shirley Meng of UC San Diego; Bao Qiu,* Yonggao Xia and Zhaoping Liu of the Chinese Academy of Sciences, Zhejiang, China; Lijun Wu* and Yimei Zhu of Brookhaven National Laboratory; Jun Wang of University of Müenster; and Yan Chen and Ke An of Oak Ridge National Laboratory. The work performed in the United States was supported by grants from the Department of Energy.
*These authors contributed equally to this work.
Share This:
You May Also Like
Stay in the Know
Keep up with all the latest from UC San Diego. Subscribe to the newsletter today.


