
Stuck on Flu
How a sugar-rich mucus barrier traps the virus – and it gets free to infect
By:
- Scott LaFee
Published Date
By:
- Scott LaFee
Share This:
Article Content
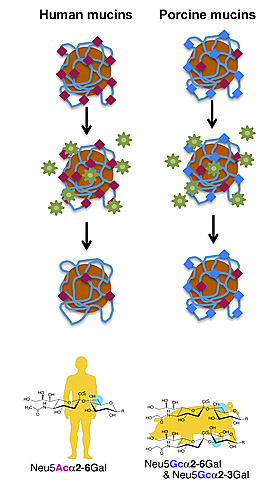
In this cartoon, experimental magnetic beads are coated with human or pig mucins (grey mesh), which are proteins containing sialic acids (red or blue diamonds), part of a protective mucus net secreted by respiratory cells. Humans and pigs have different sialic acids on their mucins, as indicated by the bottom molecular structures. The flu virus (green stars) bind to and cleave off sialic acids, snipping through the host mucus net to infect cells.
Researchers at the University of California, San Diego School of Medicine have shown for the first time how influenza A viruses snip through a protective mucus net to both infect respiratory cells and later cut their way out to infect other cells.
The findings, published online today in Virology Journal by principal investigator Pascal Gagneux, PhD, associate professor in the Department of Cellular and Molecular Medicine, and colleagues, could point the way to new drugs or therapies that more effectively inhibit viral activity, and perhaps prevent some flu infections altogether.
Scientists have long known that common strains of influenza specifically seek and exploit sialic acids, a class of signaling sugar molecules that cover the surfaces of all animal cells. The ubiquitous H1N1 and H3N2 flu strains, for example, use the protein hemagglutinin (H) to bind to matching sialic acid receptors on the surface of a cell before penetrating it, and then use the enzyme neuraminidase (N) to cleave or split these sialic acids when viral particles are ready to exit and spread the infection.
Mucous membrane cells, such as those that line the internal airways of the lungs, nose and throat, defend themselves against such pathogens by secreting a mucus rich in sialic acids – a gooey trap intended to bog down viral particles before they can infect vulnerable cells.
“The sialic acids in the secreted mucus act like a sticky spider’s web, drawing viruses in and holding them by their hemagglutinin proteins,” said Gagneux.
Using a novel technique that presented viral particles with magnetic beads coated with different forms of mucin (the glycoproteins that comprise mucus) and varying known amounts of sialic acids, Gagneux and colleagues demonstrated that flu viruses counteract the natural barrier by also using neuraminidase to cut themselves free from binding mucosal sialic acids.
More notably, he said that by blocking neuraminidase activity in the mucus, the viruses remain stuck. “They can’t release themselves from the mucus decoy and thus can’t infect.”
The discovery is likely to alter the way researchers and pharmaceutical companies think about how viruses and flu therapies function. Existing drugs like Tamiflu and Relenza inhibit neuraminidase activity and presumably dampen the ability of the flu virus to spread among cells. The work by Gagneux and colleagues suggests inhibiting neuraminidase activity in mucus may reduce the initial risk of infection.
The challenge will be to restrict neuraminidase inhibition to the mucus. Many types of cells in the human body produce neuraminidases, each performing vital cellular functions, particularly in the brain. Limiting neuraminidase inhibition to relevant mucus-secreting cells is necessary to reducing potential side effects.
“The airway’s mucus layer is constantly being shed and renewed, within a couple of hours the entire layer is replaced by a new layer,” said first author Miriam Cohen, PhD, an assistant project scientist in Gagneux’s lab. “A drug or compound that slows down neuraminidase activity rather than completely inhibit its activity will suffice to enhance the natural protective effect of mucus and prevent infection.”
Co-authors include Hooman P. Senaati and Nissi M. Varki, Department of Cellular and Molecular Medicine, UCSD; Xing-Quan Zhang and Robert T. Schooley, Division of Infectious Disease, UCSD; Hui-Wen Chen, Division of Infectious Disease, UCSD and School of Veterinary Medicine, National Taiwan University.
Funding support for this study came, in part, from the University of California Laboratory Fees Research Program, The G. Harold and Leila Y. Mathers Charitable Foundation, the National Institute of Allergy and Infectious Diseases (1UO1A1074521), the National Institute of Neurological Disorders and Stroke (P30 NS047101) and UC San Diego Cancer Center Specialize Support grant P30 CA23100.
Share This:
Stay in the Know
Keep up with all the latest from UC San Diego. Subscribe to the newsletter today.


