
UC San Diego Nanoengineer Selected as the U.S. Nominee for 2017 ASPIRE Prize
Published Date
By:
- Liezel Labios
Share This:
Article Content

Liangfang Zhang
Nanoengineering professor Liangfang Zhang at the University of California San Diego has been selected as the U.S. nominee for the APEC Science Prize for Innovation, Research and Education (ASPIRE). Zhang won the nomination for his revolutionary work in the field of nanomedicine, which focuses on nanomaterials for medical applications.
The ASPIRE Prize is an annual award hosted by the Asia Pacific Economic Cooporation (APEC) that recognizes young scientists under the age of 40 who have demonstrated a commitment to excellence in scientific research and cross-border research. Each APEC member economy identifies its own nominee for the prize. The U.S. State Department’s Office of Science and Technology Cooporation, in conjunction with Wiley and Elsevier, holds an annual competition to select a scientist to represent the United States on the APEC stage. This year, the U.S. ASPIRE competition received a record number of applicants, and the winner was chosen by a panel of scientific experts throughout the U.S. government.
Zhang will receive a $3,000 cash prize and be recognized in an award ceremony in June in Washington DC. As the U.S. ASPIRE nominee, Zhang will compete against nominees from other APEC economies for the APEC-wide ASPIRE Prize which includes a $25,000 prize.
The theme for the 2017 ASPIRE Prize is “New Material Technologies.” This interdisciplinary theme focuses on how advanced materials and processing technologies are used to drive scientific innovation.
“It’s a great honor to be selected as the U.S. representative for the ASPIRE Prize. It means that my lab’s research in biomaterials and nanomedicine is making a big impact,” Zhang said.
New Materials from Cells
Zhang invented an ingenious way to make nanoparticles perform therapeutic tasks in the body, like treat injuries and deliver drugs to specific sites, without being rejected by the immune system. His idea was to disguise synthetic nanoparticles as the body’s own cells—human red blood cells, platelets, beta cells and others—by coating them with natural cell membranes from the body. His cutting-edge work based on this idea has earned him various honors, including recognition in Popular Science magazine’s annual “Brilliant 10” list and MIT Technology Review’s annual 35 Innovators Under 35 list.
“We’re essentially camouflaging nanoparticles to look and act like they belong in the body. We want to mimic the natural interaction of the body’s cells with the immune system in order to make new biomimetic nanoparticles that can safely function and survive in the body for long periods of time,” Zhang said.
Zhang’s cell membrane coating technology marked a major breakthrough in the field of materials design. Trying to mimic the most important properties of a human cell membrane in a synthetic coating requires an in-depth biological understanding of how all the proteins and lipids function on the surface of a cell. It also poses what many researchers consider an insurmountable technical challenge—recreating that same cell surface precisely in the lab. But Zhang’s approach was to just take the whole surface membrane from an actual cell.
“We approached this problem using an engineering shortcut and bypassed all of this fundamental biology and these technical challenges,” Zhang said. “We don’t need to fully understand exactly what is going on at the protein level. We can just take the entire cell membrane, coat it onto a nanoparticle surface, and make the nanoparticle look like that cell.”
Red Blood Cell Masquerade
Zhang’s cell membrane coating technology made its debut in a 2011 study, in which his team showed a new way to disguise nanoparticles as red blood cells. The method involved collecting the membranes from red blood cells and wrapping them around biocompatible polymeric nanoparticles.
This work was an important first step toward a nanodevice—for applications like drug delivery—that could circulate in the body for extended periods without being attacked by the immune system. Since red blood cells live in the body for up to 120 days, Zhang reasoned they would be good models and resources for making long-circulation drug delivery nanodevices.
In the 2011 study, Zhang’s team showed that nanoparticles coated with red blood cell membranes circulated in the bodies of mice for up to two days. This was an improvement over other nanoparticle systems developed for drug delivery—these are coated with a synthetic material made to temporarily suppress immune recognition and circulate in the body for just a few hours. Zhang explained that a major concern for the synthetic coating is that, after repeated use, it will eventually trigger an immune response and in the long run, these types of drug delivery systems could be drastically less effective.
And this red blood cell disguise offers more than just extended circulation time in the body. Because red blood cells are one of the primary targets of pore-forming toxins, such as those produced by MRSA (methicillin-resistant Staphylococcus aureus), Zhang reasoned that his faux red blood cells could also serve as decoys to lure these toxins away.
Pore-forming toxins work by puncturing a red blood cell’s membrane, forming pores that cause the cell to essentially leak to death. But when toxins attack a red blood cell membrane draped over a nanoparticle, they get trapped and can’t go on to attack the body’s own red blood cells.
Zhang’s team showed that these toxin-absorbing “nanosponges” were capable of sopping up MRSA toxins in the bloodstream of mice and diverting them away from their cellular targets. And by removing the toxins, the nanosponges helped clear up infections caused by MRSA bacteria.
“The toxins act as a defense shield for the bacteria, making it harder for the immune system to fight back. Remove this shield, and the bacteria become more vulnerable. With the nanosponges, we essentially have a new way to combat hard-to-treat bacterial infections—without the use of antibiotics,” Zhang said.
Closetful of Cellular Cloaks
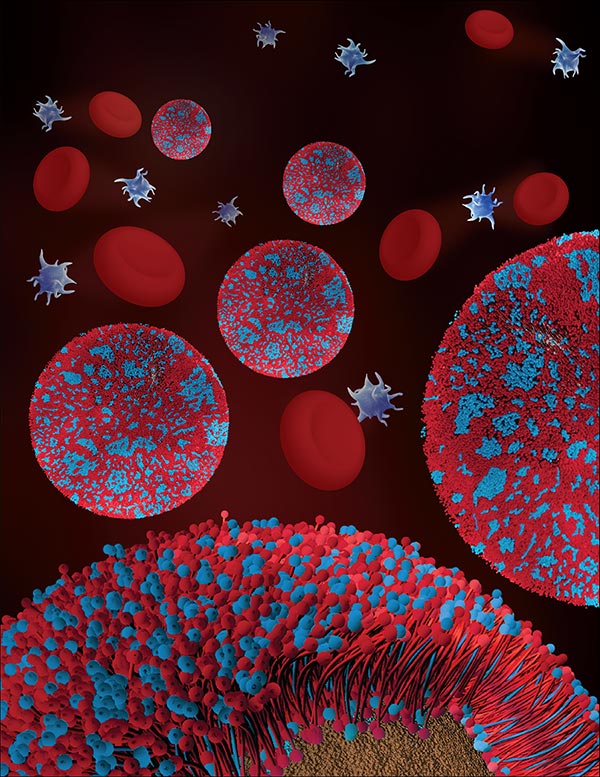
Illustration of cell membrane coated nanoparticles is featured as the inside front cover for the April 25, 2017 issue of Advanced Materials. Image courtesy of Nanomaterials & Nanomedicine Laboratory at UC San Diego
Over the past six years, Zhang and his lab have taken their cell membrane coating technology to new heights. They’ve disguised nanoparticles as human platelets, which have a natural affinity for binding to damaged blood vessels and certain pathogens in the body, like MRSA bacteria. Because of this affinity, platelet-mimicking nanoparticles could be used for targeted drug delivery.
Zhang’s team conducted several experiments. In one, they packed platelet membrane coated nanoparticles with a drug used to heal damaged arteries and administered them to wounded rats; in another experiment, they packed the nanoparticles with antibiotics and administered them to mice infected with MRSA bacteria. In both cases, the drugs were delivered primarily to the affected areas. “That shows the power and the promise of targeted delivery,” Zhang said.
Zhang’s team has also made disguises out of the membranes of beta cells, which are insulin-producing cells in the pancreas. They coated a nanofiber with beta cell membranes to create a pancreas-like microenvironment that encouraged beta cells to congregate, grow and produce more insulin. This work could lead to new treatments for patients with diabetes. “This is another example of mimicking natural interactions in the body to create more effective therapies,” Zhang said.
Recently, Zhang demonstrated a new direction in materials design—making the first hybrid cell membrane coated nanoparticles. In a study published this month in Advanced Materials, Zhang and his team showed they could mix two different cell membranes (red blood cell and platelet) and fuse them to form a hybrid membrane as a new type of coating for nanoparticles. The result is a nanoparticle that has the long-circulation, toxin-absorbing properties of a red blood cell combined with the targeting functions of a platelet cell. And Zhang says the possible hybrid cell membrane combination is not limited to red blood cell and platelet membranes, it can be any mix of cell membranes. “This is a proof of concept for making multifunctional nanoparticles.”
But Zhang and his research group are not stopping there. Next on their list is using cell membrane coating technology to develop new systems for combating cancer. Zhang is also working with several biopharmaceutical companies in San Diego to get the cell membrane coated nanoparticles into human clinical trials.
Share This:
You May Also Like
Stay in the Know
Keep up with all the latest from UC San Diego. Subscribe to the newsletter today.


